Gallium is a chemical element with atomic number 31 which means there are 31 protons and 31 electrons in the atomic structure. The chemical symbol for Gallium is Ga. Gallium has similarities to the other metals of the group, aluminium, indium, and thallium. The atomic number A: equal to the number of protons (placed as a left subscript) 3. The mass number Z: equal to the number of protons and neutrons in the isotope (placed as a left superscript) Examples 1: Consider two isotopes of gallium, one having the 37 neutrons and the other having 39 neutrons. Gallium is a chemical element with the symbol Ga and atomic number 31. Elemental gallium is a soft, silvery metal at standard temperature and pressure; however in its liquid state it becomes silvery white. 31: Gallium - Gallium Ga Group: 13 Period: 4 Atomic number: 31 Atomic mass: 69.723 Configuration: Ar 3d 10 4s 2 4p 1 Atomic radius: 136 pm Covalent radius: 122 pm Electron affinity: 28.9 eV Ionization energy: 5.9993 eV Electronic term: 2 P 1/2 Mass fraction in the earth crust: 0.000019 Mass fraction in the earth space: 0.00000001.
Gallium is the chemical element with the atomic number 31 and symbol Ga on the periodic table. It is in the Boron family (group 13) and in period 4. Gallium was discovered in 1875 by Paul Emile Lecoq de Boisbaudran. Boisbaudran named his newly discovered element after himself, deriving from the Latin word, “Gallia,” which means “Gaul.” Elemental Gallium does not exist in nature but gallium (III) salt can be extracted in small amounts from bauxite and zinc ores. Also, it is known for liquefying at temperatures just above room temperature.
Introduction
Gallium is one of the elements originally predicted by Mendeleev in 1871 when he published the first form of the periodic table. He dubbed it ekaaluminum, indicating that it should have chemical properties similar to aluminum. The actual metal was isolated and named (from the Latin Gallia, for France) by Paul-Emile Lecoq de Boisbaudran in 1875.
The detective work behind the isolation of gallium depended on the recognition of unexpected lines in the emission spectrum of a zinc mineral, sphalerite. Eventual extraction and characterization followed. Today, most gallium is still extracted from this zinc mineral.
Although once considered fairly obscure, gallium became an important commercial item in the '70s with the advent of gallium arsenide LEDs and laser diodes. At room temperature gallium is as soft as lead and can be cut with a knife. Its melting point is abnormally low and it will begin to melt in the palm of a warm hand. Gallium is one of a small number of metals that expands when freezing.
Basic Chemical and Physical Properties
| Atomic Number | 31 |
| Atomic Mass | 69.723 g/mol |
| Element Category | Post-transition metal |
| Phase | Solid |
| Electronegativity | 1.6 (Pauling Scale) |
| Density (at 0oC) | 5.91 g/cm3 |
| Melting Point | 29.7646oC |
| Boiling Point | 2204oC |
| Atomic Radius | 135 pm |
| Ionic Radius | 62 pm |
| Isotopes | 2 (69Ga; 60.11% & 71Ga; 39.89%) |
| 1st ionization energy | 578.8 kJ/mol |
| Electrode Potential | -0.56 eo |
| Electrical Conductivity | 9.1 |
| Oxidation States | +3,+2, +1 |
| Hardness | 1.5 (Mohs) 60 MPa (Brinell) |
| Crystal Structure | Orthorhombic |
| Specific Heat | 25.86 J/molK |
| Heat of Fusion | 5.59 kJ/mol |
| Heat of Vaporization | 254 kJ/mol |
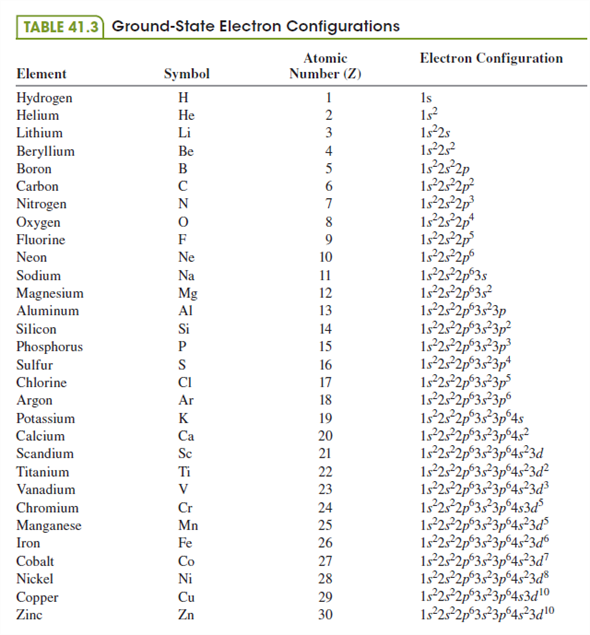
| Electronic Configuration | 1s22s22p63s23p64s23d104p1 [Ar]4s2 3d104p1 |

Characteristics
Gallium has a few notable characteristics which are summarized below:
- In its solid phase, Gallium is blue-grey in color
- It melts in temperatures warmer than room temperature; therefore, if you were to hold a chunk of gallium in your hand, it will start to liquefy.
- Solid gallium is soft and can easily be cut with a knife.
- It is stable in air and water, but reacts and dissolves in acids and alkalis.
- If solidifying, gallium expands by 3.1 percent and thus storage in glass or metal is avoided.
- It also easily to transform into an alloy with many metals and has been used in nuclear bombs to stabilize the crystal structure.
- Gallium is one of the few metals that can replace the use the mercury in thermometers because its melting point is close to room temperature.
Video 1: the video depicts the solidifying of liquid Gallium in 10x speed. Density of solid Gallium smaller than density of the liquid, so it's expanding during solidification and break the bottle.
Video 2: The video shows Gallium melting in your hands due to its melting point.
Occurrences
Gallium usually cannot be found in nature. It exists in the earth's crust, where its abundance is about 16.9 ppm. It is extracted from bauxite and sometimes sphalerite. Gallium can also be found in coal, diaspore and germanite.
Applications
Health: While Gallium can be found in the human body in very small amounts, there is no evidence for it harming the body. In fact, Gallium (III) salt is used in many pharmaceuticals, used as treatment for hypercalcemia, which can lead to growth of tumors on bones. Further, it has even been suggested that it can be used to treat cancer, infectious disease, and inflammatory disease. However, exposure to large amounts of Gallium can cause irritation in the throat, chest pains, and the fume it produces can lead to very serious conditions.
Semiconductors: Roughly 90-95% of gallium consumption is in the electronics industry. In the United States, Gallium arsenide (GaAs) and gallium nitride (GaN) represent approximately 98% of the gallium consumption. Gallium arsenide (GaAs) can convert light directly into electricity. Further, gallium arsenide is also used in LEDs and transistors.
Other applications of Gallium deal with wetting and alloy improvement:
Gallium has the property to wet porcelain and even glass surfaces. As a result, gallium can be used to create dazzling mirrors. Scientists employ an alloy with Gallium for the plutonium pits of nuclear weapons to stabilize the alloptropes of plutonium. As a result, some have issue with the element.
Number Of Protons In Gallium
References
- Petrucci, Harwood, Herring, and Madura - General Chemistry 9th Edition
Problems

- What is the electronic configuration of Gallium?
- What do you think is one of the issues that people might have with usage of gallium?
- Gallium is part of which group and period?
- What are some applications of Gallium?
- Name three properties of Gallium that make it different from any other element.
Answers
- 1s22s22p63s23p64s23d104p1
- The use of it in nuclear bombs.
- Gallium is in group 13 (Boron family) and in period 4.
- Semiconductors; cancer treatment; hypercalcemia treatment; stabilization in nuclear bombs. See section above on Application for more detail.
- 5. See the section above on properties and characteristics for more detail.
- Gallium is blue-grey in color in its solid phase.
- Melts in temperatures warmer than room temperature
- Stable in air and water, but reacts and dissolves in acids and alkalis.

Contributors and Attributions
- Angela Tang, Sarang Dave
Stephen R. Marsden
Atomic Number of Gallium is 31.
Chemical symbol for Gallium is Ga. Number of protons in Gallium is 31. Atomic weight of Gallium is 69.723 u or g/mol. Melting point of Gallium is 29,8 °C and its the boiling point is 2403 °C.
» Boiling Point» Melting Point» Abundant» State at STP» Discovery Year
About Gallium
Gallium is a rare and very soft metal of light grey color, which resembles aluminum in some of its properties. It is a semi-conductor. This chemical element has got its name after the Latin name for France, Gallia. It is quite hard to find gallium on the surface of our planet; it can be extracted from some types of coal and bauxites, but in quite small doses. Gallium has no biologic importance and is non-toxic. It makes alloys with many other metals, and these alloys can be used for some parts for mobile phones, light emitting diodes, pressure sensors, etc.
Uses of Gallium
Gallium, a silvery-white and soft metallic element with the symbol Ga, is mainly used in electronics. Most of the gallium produced in the world are employed to make Gallium arsenide. Gallium can be used in pharmaceuticals, radiopharmaceuticals, and medicine too. Gallium compounds are used in rectifiers, transistors, solar panels, mobile phones, and optoelectronic nanosystems. Gallium arsenide, a compound of gallium and arsenic with the formula GaAs, is used as a semiconductor and employed to manufacture LED TVs, lasers, microwave integrated circuits, solar cells, etc. Gallium nitride, an alloy of gallium and nitrogen with the formula GaN, is also used as a semiconductor. It is employed in mobile phones, Blu-ray technology, LEDs, etc.
Where To Buy Gallium
Compounds with Gallium
- Ga2O: Gallium(I) oxide
- GaS: Gallium Sulfide
- GaSe: Gallium Selenide
- GaTe: Gallium(II) telluride
- Ga(OH)3: Gallium(III) hydroxide
- GaAs: Gallium arsenide
- GaN: Gallium nitride
- AlGaAs: Aluminium gallium arsenide
- GaP: Gallium phosphide
- GaSb: Gallium antimonide
- InGaAs: Indium gallium arsenide
- GaAsP: Gallium arsenide phosphide
- AlGaN: Aluminium gallium nitride
- InGaN: Indium gallium nitride
Atomic Number Of Gallium
Properties of Gallium Element
| Atomic Number (Z) | 31 |
|---|---|
| Atomic Symbol | Ga |
| Group | 13 |
| Period | 4 |
| Atomic Weight | 69.723 u |
| Density | 5.907 g/cm3 |
| Melting Point (K) | 302.9146 K |
| Melting Point (℃) | 29,8 °C |
| Boiling Point (K) | 2673 K |
| Boiling Point (℃) | 2403 °C |
| Heat Capacity | 0.371 J/g · K |
| Abundance | 19 mg/kg |
| State at STP | Solid |
| Occurrence | Primordial |
| Description | Metal |
| Electronegativity (Pauling) χ | 1.81 |
| Ionization Energy (eV) | 5.9993 |
| Atomic Radius | 130pm |
| Covalent Radius | 126pm |
| Van der Waals Radius | 187 |
| Valence Electrons | 3 |
| Year of Discovery | 1875 |
| Discoverer | Lecoq de Boiskaudran |
What is the Boiling Point of Gallium?
Gallium boiling point is 2403 °C. Boiling point of Gallium in Kelvin is 2673 K.
What is the Melting Point of Gallium?
Gallium melting point is 29,8 °C. Melting point of Gallium in Kelvin is 302.9146 K.
How Abundant is Gallium?
Abundant value of Gallium is 19 mg/kg.
What is the State of Gallium at Standard Temperature and Pressure (STP)?
Atomic Number Of Gallium Arsenide
State of Gallium is Solid at standard temperature and pressure at 0℃ and one atmosphere pressure.
When was Gallium Discovered?
Gallium was discovered in 1875.
Gallium Mass Number
